
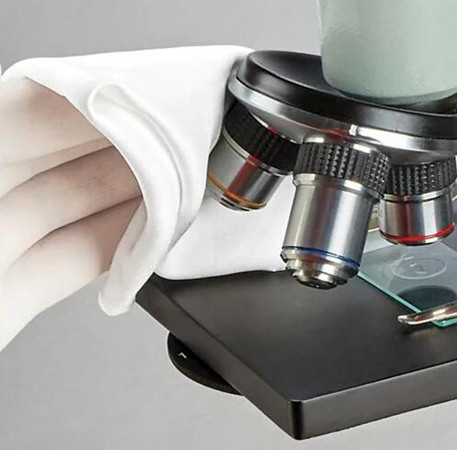
A quick determination of a stencil wiping roll’s ability to withstand fiber detachment and consequent contamination of circuit boards during the printing process. The goal of this test is to select an SMT stencil cleaning roll that is free of weak linty paper fibers that can break ways. Using a lint-free roll such as the Jiedao's Cleanroom stencil wiper rolls can help prevent damage to equipment, defects, bridges, blocked apertures, low transfer efficiency, and solder contamination. Contaminated PC boards are a common problem. While the contamination itself is often thought of as a mystery, the cause may actually come from the stencil wiping roll itself. This is largely due to the sticky solder paste and sharp edges of the stencil’s apertures. The stencil wiping roll is supposed to clean the stencil in an attempt to prevent contamination. However, some stencil wiping rolls often leave behind pieces of its material on the stencil, subsequently contaminating the PC board. When these contaminants pass through the oven, voids will occur. One can determine the probability of contamination with an easy test of the stencil wiping roll. The test is called the “Tape Test”. It is a quick and simple experiment that will save any company money by not having to repair contaminated boards. It will also help any company in determining which stencil wiping rolls are acceptable. Stencil Cleaning Roll Tape Test Procedure 1.First, cut off a piece of scotch tape or black electrical tape about 2 inches in length. 2.Place the strip of tape along the surface of the stencil wiping fabric. 3.Once the tape has adhered to the surface, pressure must be applied. The pressure should be applied in an even fashion. 4.To ensure that the pressure is equally applied roll a 1,000gm calibration weight over the taped surface. If a weight of this size is not available, a seam pro (used for wallpaper seam attachment), a full can of soda or any tool that will provide equal weight distribution can be used. In some cases, simply using a finger will be acceptable. 5.If Scotch tape is used, place it on a black surface and observe the mount of fibers pulled off with the naked eye or better yet a magnifier. This test can easily be quantified by counting fibers and particles under magnification. 6.For the best determination, repeat this step for front and back sides of the wiping rolls. Important! This test is only valid when comparing fabrics.
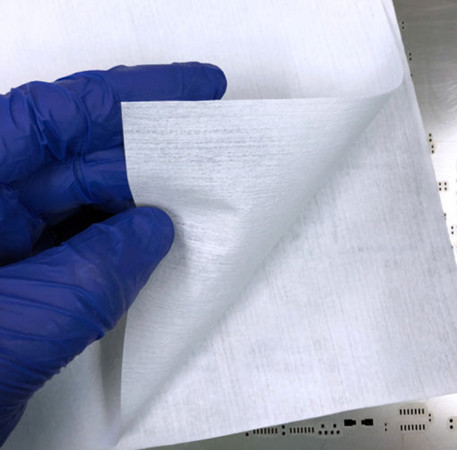
You can see various wipers in the world to control contamination at cleanroom. You need to know what makes an ideal wipe for its specific purpose. Examining two essential wiper attributes (material and construction) is 1st step in determining if wiper is proper for specific situation and application. Material: Synthetics, Cotton and Blend Synthetics like polyester and nylon are cleaner than cotton and also possess a wider range of chemical resistances. They are hydrophobic meaning that they do not naturally absorb and hold water or aqueous-based liquid. That said washing treatment of synthetics can significantly increase absorbency capabilities. Cotton and Blend are hydrophilic, naturally absorbent, strong, and can withstand high temperatures. However making cotton absorbent may increase fiber shading during wiping applications. Also typically polyester and cellulose are paired to offer the cleanliness of a synthetic with hydrophilic properties of a natural material. However, blends are open limited to general cleaning or maintenance usage in supporting areas for more critical environments. Construction of Substrates: Non-woven, Knit and Composite. Nonwoven cleanroom wipers substrate can be made of natural, synthetic fiber and filaments that are turned into a homogeneous sheet with chemical, thermal, mechanical or solvent treatment. Non-woven is a great choice for you to trim consumables costs for less critical environment. Knitted substrates are made of interlaced loops of yarn. Polyester is the predominant base material for knitted wipers. They can be combined into multiple layers through a variety of method which further increase absorbency and durability. Composite wipers are multiple substrate layers fused into one sheet, for wipers, cellulose is typically sandwiched between layers of polypropylene. Although composite offer a potentially broad range of use than a wiper of either individual substrate, their overall applicability to environments and process will always be limited by the least compatible material.

This article covers cleanroom validation for an ISO 7 medical device cleanroom (Class 10,000). There are many different manufacturing standards associated with each different class of device and its end-use requirements. ISO validation for a non-sterile medical device cleanroom does not mirror a cleanroom for sterile counterparts. It is the responsibility of the manufacturer to establish and validate the appropriate manufacturing processes, procedures, materials, and environments for a specific device. In previous parts of this cleanroom build guide, we’ve covered each step of the cleanroom construction process including: Medical Device Cleanroom Build Guide Part 1: Walls, Windows, & Containment Part 2: Electrical, Mechanical & Partitions Part 3: Fan Filter Units – Ceiling Grids Part 4: VCT Cleanroom Flooring Part 5: HVAC Design & Fan Filter Integration Part 6: Pressure Differentials, Humidity and Temperature Calibration Part 7: Cleanroom ISO Class Validation As-Built and At-Rest Cleanroom Differences ISO 7 Clean Room Production Area for Medical Devices Above we see the primary production area of an ISO 7 medical device cleanroom (ISO 7 cleanroom area). The room is considered the equivalent of a class 10,000 (Class 10K) room by previous FED-STD-209 E cleanroom standards. As-Built Cleanrooms The cleanroom is now considered “As-Built”. As-Built Cleanroom: Complete and ready for operation, with all services connected and functional, but without equipment or operating personnel. At-Rest Cleanroom: Complete with all services functioning and with equipment installed and operating, as specified, but without operating personnel present. Medical Device Cleanroom Validation A well designed and diligently constructed cleanroom will meet every performance criteria for cleanroom validation after calibration. The PAC cleanroom pictured here required no adjustment and tested out at ISO 7 in all areas of the cleanroom. What Happens the Cleanroom is Actually Cleaner than Required? Most cleanroom designers over-spec fan filter requirements for a couple of reasons: A) Fan filter blowers produce more noise and vibration at 100% capacity B) Forward impeller fan filter designs run most efficiently at ~50% power C) Filter media requires less frequent replacement at lower throughput rates D) A single fan filter unit malfunction is less likely to compromise the room as a whole E) Under-powered fan filter units become obsolete more quickly Overcoming Efficiency Loss with Flexible Fan Filter Control Systems HVAC is the most costly aspect of cleanroom operation: the HVAC system runs 24 hours a day, 7 days a week. Over-clocked cleanroom performance is best matched with a flexible fan filter control system. Here, the gowning room only requires an ISO 8 airflow and particulate sampling threshold. So, how do we get best-case performance without sacrificing energy efficiency? A cleanroom equipped with a smart fan filter system allows fan speed adjustment for any part...

What Is A Cleanroom Standard? Standards organizations define high-detail techniques and scientifically backed approaches towards “standardized” measurements and tests. The most common standards organizations for cleanroom manufacturing include ASTM, IEST, and ISO. Cleanroom Wipe Evaluation Quick Links — Particulate | Fiber Testing Methods — Ionic Contamination, Trace Metals & Heavy Metal Contamination — Nonvolatile Residue – NVR – in Cleanrooms — Microbiological & Pyrogen Testing — Profiles for Medical Device Wipedown & Electronics — Wipe Testing Standards for ASTM, IEST & ISO Organizations ASTM Cleanroom Wipe Tests & Standards ASTM (American Standards for Testing and Measurement) is an organization representing the collective expertise and judgment of stakeholders from industry, government, academia, trade groups, consumers, and more. ASTM International standards are known for establishing high quality best practices, measurement methods, and technique validation for repeatable manufacturing outcomes. “2,000 subcommittees) meet face-to-face and virtually, using tools like electronic balloting and online collaboration areas, to develop standards. ASTM International publishes those standards shortly thereafter.” ASTM Tests for Cleanroom Wiper Materials Examples ASTM E 2090, Standard Test Method for Size Differentiated Counting of Particles and Fibers Released from Cleanroom Wipers Using Optical and Scanning Electron Microscopy ASTM E 1560, Standard Test Method for Gravimetric Determination of Nonvolatile Residue from Cleanroom Wipers ASTM F 50, Standard Practice for Continuous Sizing and Counting of Airborne Particles in Dust Controlled Areas and Clean Rooms Using Instruments Capable of Detecting Single Sub Micrometre and Larger Particles ASTM E 2090, Standard Test Method for Size Differentiated Counting of Particles and Fibers Released from Cleanroom Wipers Using Optical and Scanning Electron Microscopy EN 1276 for bacterial efficacy, EN1650 for fungal efficacy. EN 13704 for sporicidal efficacy. IEST Cleanroom Wipe Test Methods & Standards IEST (Institute for Environmental Sciences and Technology) is a standards organization that outlines industrial benchmarks for assessing cleanroom wiper performance, test methods, and validation benchmarks. IEST-RP-CC016, The Rate of Deposition of Nonvolatile Residue in Cleanrooms IEST-STD-CC1246, Product Cleanliness Levels and Contamination Control Program IEST-RP-CC003, Garment System Considerations for Cleanrooms and Other Controlled Environments IEST-RP-CC004: Evaluating Wiping Materials Used In Cleanrooms And Other Controlled Environments is one of the most common cleanroom methodologies for task wiper contamination testing and benchmarks. Read: Complete Guide to IEST Test Methods for Cleanroom Wipes and Fabrics ISO Class Cleanroom Wipe Requirements Establishing proper ISO rated products is important for a minimal cleanroom product assessment, but not sufficient. ISO standards regar...

Question: What Class clean room is required to manufacture FDA approved N95 masks, isolation gowns, surgical masks, etc? There are various benchmarks for FDA approved products such as surgical N95 masks and surgical isolation gowns. Before we get to the answer, lets look more closely at what types of products and devices are FDA regulated. Does the FDA Regulate PPE? Some PPE items are regulated by the FDA, others are not. Naturally, any item label, approved, and specified as a medical device is approved and regulated by the FDA. Some N95 mask are certified for healthcare and surgical use and thus require more elaborate production controls. Further, some face masks require “cleanroom rated” materials which will not shed lint or fibers. Does the FDA Regulate N95 Masks? The FDA regulates products such as surgical N95 respirators and surgical-grade gowns. (Considered medical devices). Masks and PPE for non-medical purposes are not medical devices and are not regulated by the FDA. FDA Cleanroom Standards for PPE Production In general, the FDA does not provide specific guidance on cleanroom build specifications or requirements. They will defer to USP or CGMP standards. Most USP and CGMP cleanroom build requirements are based on ISO 14644-1, a baseline where we start all of our cleanroom builds. Cleanroom Requirements for Sterile vs Non Sterile Devices Something like an intracavity device or other sterile medical device may require an ISO 5 cleanroom, whereas medical device packaging and other less sensitive processes are usually specified at ISO 7 or ISO 8. Cleanroom ISO Class for Sterility Assurance It really depends on how the final device will be classified, labeled, what it will be used for, and what degree of sterility assurance it requires (if any). For example, the desired Sterility Assurance Level (SAL) for garments to be used in sterile pharmaceutical manufacturing is 10^6, which translates into an assurance that a sterile garment has only one-in-one million probability of being non-sterile. ISO class is just one aspect of delivering a production ready cleanroom. Sterile procedures normally demand cascading cleanroom suites of positive pressure, air quality monitoring, precise pressure differentials, aggressive cleaning regimens, and air tight seals. Terminally Sterile Device Manufacturing If you’re looking to manufacture anything terminally “sterile”, like sterile surgical gowns or masks, you’d spec an ISO 5 cleanroom at the least. (ISO 5 is the general standard of a “sterile” or aseptic workflow environment. For items that will be sterilized after production, an ISO 7 medical device cleanroom is a common benchmark. Clean Rooms for Sterile Products An ISO 5 room would be similar in nature to cleanrooms built under USP 797 (sterile) or USP 795 (non-sterile) standards. The room has a cascading suite of positive pressure rooms which start at an ISO 7 or 8 buffer / ante for gowning and product preparation, while the critical production proce...

Cleanroom wipes and gloves undergo LAL and Agar Overlay Analysis when testing for pyrogen and endotoxin levels. These tests ensure that cleanroom materials such as wipes or PPE do not introduce bacteria or pathogens into a cleanroom manufacturing process. This post details the methodology, historical relevance, and modern cleanroom solutions for pyrogen-free and low-endotoxin gloves and wipes. Biological Contamination – Pharmaceuticals – Sterile Compounding – Lifesciences Medical device and pharmaceutical cleanroom environments must address environmental exposure to bacteria, fungi, or viruses. Each common tool, material, or surface is capable of retaining microbiological artifacts, some inert, others parthenogenic. Medical Devices, Pharmaceuticals, and Healthcare Wipe Contamination In medical device and pharmaceutical cleanroom environments, providers must address environmental exposure to bacteria, fungi, or viruses. Repeated sterile outcomes are critical for applications such as sterile compounding, but also for test and research environments in laboratories and test facilities. Every surface and tool is subject to a pragmatic and well-documented cleaning regimen that requires aggressive sterilants and certifiable products such as EPA registered, one-step disinfectants. Example Wipedown Method for a Biological Safety Cabinet Decontaminating critical tools or surfaces requires a multistep process with hyper-clean wipes, disinfectant agents, and sterile rinse water. What are Pyrogens and Endotoxins? Pyrogens Pyrogens include endotoxins which are derived from Gram-negative bacteria. Non-endotoxin pyrogens result from chemicals or microorganism-derived substances (source). Endotoxins “Bacterial endotoxins, also known as lipopolysaccharides, are a fever-producing by-product of gram-negative bacteria commonly known as pyrogens”. (Source) Endotoxins are toxins released after the destruction of a host cell. Some endotoxins are classified as pyrogens. Pyrogens are molecules that produce fever.
Categories
New Products
Cleanroom Wipers For Screen Cleaning Fiber Lint Free Cleanroom Wipes Dust Free Read More
Polyester Spunlace Double Knit Nonwoven Cleanroom Wipes Read More
Polyester Nylon Knitted Microfibre / Microdenier Wiper Read More
Direct Manufacturer Factory Selling Nonwoven Wiper Rolls Industrial Wiper Rolls Read More
Copyright © 2024 Nanan Jiedao Electronic Material Co.,Ltd.. All Rights Reserved. Powered by

IPv6 network supported