

Where is the threshold for the production of medical masks? To produce masks that meet medical standards, it is necessary to have quality raw materials. General civilian masks can be produced with only two layers of spunbond non-woven fabrics + filter layers, while medical masks and N95 masks need to replace the middle filter layer with melt-blown non-woven fabrics. The melt-blown non-woven fabric itself has an electrostatic adsorption effect. With this layer of melt-blown non-woven fabric, the droplets and viruses in the air will be attracted by static electricity, and ordinary masks can be turned into "medical surgical masks" or "medical protective masks". ". To produce this melt-blown non-woven fabric, melt-blown material is required, and melt-blown material is a chemical product that requires polypropylene synthesis. The small workshop has no non-woven fabrics, and no meltblown materials. It can only use inferior fabrics or even pulp to make Sanwu fake masks. Although the output is large, the only use of the products may be to cover the faces of big stars. On the other hand, medical masks belong to the second category of medical devices, and must be qualified for the production of medical devices. Although the country has opened the green channel for application in special periods, it is difficult for ordinary small factories to obtain the corresponding production qualifications. The materials are exquisite, the qualifications are difficult to obtain, and the craftsmanship is not simple. Disposable medical masks are divided into sterile and non-sterile types. At this time, of course, all that are needed are sterile. Medical surgical masks and medical protective masks are sterile by default. Sterile masks must be produced in a clean workshop of no less than 100,000 grades. Building a clean dust-free workshop is a technical task. Even if there is a desire to build a dust-free workshop, it is difficult for companies without relevant experience to do it in half an hour. After the medical masks are produced, they are also sterilized with ethylene oxide, but in this way, there will be ethylene oxide residues on the masks, which are harmful to the human body. The analysis process takes 7 days to half a month. This is also the reason why the factory is now fully on fire, but the number of masks on the market does not seem to increase - we will not see the current production in 7 days at the earliest.

A clean room is also called a clean workshop, a clean room, or a clean room. It refers to the removal of pollutants such as particles, harmful air, bacteria, etc. , Indoor pressure, airflow speed and airflow distribution, noise vibration and lighting, static electricity are controlled within a certain demand range, and a specially designed room is given. That is to say, no matter how the external air conditions change, the interior can maintain the characteristics of cleanliness, temperature, humidity and pressure that were originally set. Clean room, also known as clean room, clean room, clean room or clean room. The main function of a clean room is indoor pollution control. Without a clean room, it is impossible to mass-produce pollution-sensitive parts. In FED-STD-2, a cleanroom is defined as a room with air filtration, distribution, optimization, construction materials and devices, in which specific regular operating procedures are used to control the concentration of airborne particulates to achieve an appropriate level of particulate cleanliness . The cleanliness of the clean room and the continuous stability of pollution control are the core standards for testing the quality of the clean room. The standard is divided into several grades according to factors such as the regional environment and degree of purification. Commonly used are international standards and domestic regional industry standards. Some well-known In addition to the general standards, the purification engineering company also has its own purification indicators that are higher than the international general standards, and its purification capacity and environmental adaptability far exceed those of international brands. Generally, industrial aluminum (stainless steel square pass, plastic-sprayed square pass) is used as the frame, and the fan filter unit FFU is used to supply air. Anti-static vertical curtains are used around it. Up to 100-100,000 grades; especially suitable for areas in the workshop that require high cleanliness, such as the high-precision product assembly area in the assembly line operation area. The main function of the dust-free workshop is to control the cleanliness and temperature and humidity of the atmosphere that the products (such as silicon chips, etc.) come into contact with, so that the products can be produced and manufactured in a good environmental space. This space is called dust-free. workshop. Dust-free paper must be used in clean and dust-free workshops.

After you’ve dedicated your time and resources to outfit your cleanroom with the best materials and equipment, you want to make sure to maintain an exceptionally clean environment so your cleanroom can function at peak performance. A lot of factors go into making sure your cleanroom is as clean as possible, from the products you use to the staff that use them. No matter what class rating your cleanroom has, cleaning your cleanroom will ensure longevity and improve efficiency. All cleanrooms require continual maintenance to be able to operate their best. Cleanrooms should be cleaned according to a regular schedule, meeting daily and weekly tasks. INDUSTRY STANDARDS VARY Cleanrooms vary widely in use. Manufacturing cleanrooms don’t have the same functions or standards as pharmaceutical or laboratory cleanrooms. Depending on the industry you’re in, your cleanroom will have a specific layout, ISO rating, and cleanliness standard. Therefore, its cleaning schedule and procedure will also differ. Cleanrooms with higher ISO ratings must be kept at much higher levels of sanitation to reduce the chance of interference of minute particles and contaminants. Conversely, cleanrooms with lower class ratings, while they may be less threatened by certain contaminants or smaller particle sizes, still require regular cleaning to maintain standards and efficiency. PREVENTION IS KEY The best way to keep your cleanroom clean is to follow proper sanitation techniques before entering a cleanroom. These include things like washing and drying hands completely, using sterile and not powdered gloves, following the proper gowning procedure for your ISO class, and making sure that all employees have access to garments and tools that fit them. In a perfect world, we would prevent contamination by introducing zero contaminants into your cleanroom environment. Of course, this is virtually impossible, which is why regular cleaning and maintenance of your cleanroom and its systems is critical. CLEANROOM CLEANING PROCEDURE: Keeping your workspace clean requires diligent adherence to daily and weekly cleaning tasks. Depending on the strictness of your class standard, more rigorous objectives may need to be added, or these tasks will need to be completed more frequently. Whatever your facility requires, create and follow a cleaning schedule that clearly defines all assignments, making them easy to understand and follow. Here are the general cleaning protocols recommended for broad cleanroom needs. DAILY CLEANROOM CLEANING Before shift begins, use a damp mop on floors and vacuum to dry. Vacuum all walls using a HEPA filter vacuum. Wash and wipe dry all windows and pass-throughs. At the end of every shift, wipe down all work areas. This may need to occur more frequently with high class standards. Put away products and supplies between shifts to prevent further contamination. WEEKLY CLEANROOM CLEANING Mop floors with a cleanroom-specific detergent, distilled water, and a HEPA filter vac...

A variety of updates were revealed including new innovative products, redesigned menu, improved navigation, request a quote functionality, and a new online customer portal. The new website includes the following enhancements: New Innovative Cleanroom Supplies: Several new products and product lines were added including disposable gloves, PPE, sterile supplies, cleanroom wipes, and cleanroom bags. Improved Layout and Navigation: The new site implements several new standardised features such as a new mega menu structure and a spacious layout for easy browsing. Request a Quote Functionality: This new feature allows customer to request a quote for large quantities or other unique requirements. Online Customer Portal: Efficiently manage resources, access contracted pricing, easily reorder and access order history. “Our new website was designed with customer experience being a number one priority. The entire Jiedao Electronics team was involved during the development stages so that we could really offer the functionality our customers deserve. We believe these upgrades make our website one of the best in the industry,” said Jack Lin, President of Jiedao Electronics. Jiedao Electronics is your partner for everything contamination control. Our goal is to work with you to reduce risks within your facility by providing solution for the entire facility, not just inside the cleanroom. For over 8 years, we have been and continue to be a leading distributor of products for a variety of tech-driven industries including Cleanroom, Pharma, Biotech, Electronics Assembly, Lab, Industrial, Aerospace, Automotive, Compounding Pharma, and Food Processing. Our extensive product line includes innovative and cost-saving solutions such as cleanroom wipes, industrial wipes, sticky mats, gloves, cleanroom apparel, static control products, and other supplies.

While we often talk about the importance of keeping contamination out of linear motion components such as linear guides and screws, when these systems are used in a cleanroom, the goal is just the opposite — to keep these components from introducing contamination into the environment. What exactly is a cleanroom? According to ISO 14644-1:2015, “Cleanroom and associated controlled environments provide for the control of contamination of air and, if appropriate, surfaces, to levels appropriate for accomplishing contamination-sensitive activities.” Cleanrooms are most commonly associated with applications in the semiconductor, electronics, and medical device industries, although other industries — such as aerospace, pharmaceuticals, and food and beverage — also use cleanroom environments in some applications. The ISO 14644-1 standard rates the level of “cleanliness” of a cleanroom on a scale from 1 (best) to 9 (worst), based on the number of particles — broken down into six size ranges — that are present in a cubic meter of air. Notice that the cleanroom standard referenced above is from the International Standards Organization (ISO). You may also see the U.S. Federal Standard 209E referenced in some instances, despite the fact that it was revoked in 2001. The FS 209E ratings can be cross-referenced to ISO ratings, but note that the class numbers don’t align. For example, a cleanroom rated as class 1 under FS 209E is rated as class 3 under ISO 14644-1.
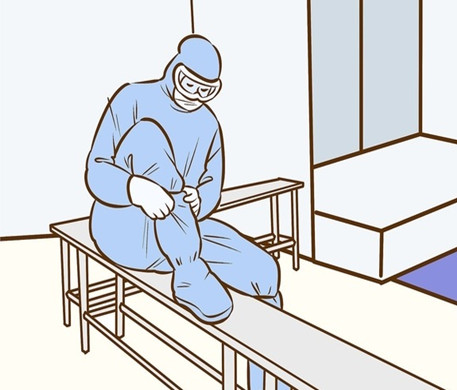
Learning how to properly put on sterile gowns is crucial to minimising the risks of contamination in any critical environment. This particularly includes cleanrooms, which are strictly designed to remain sanitary and contaminant-free (including dust and microorganisms). In addition to rigidly controlled environments, protection measures for humans are also required. Since many employees working in these cleanrooms are constantly exposed to sensitive equipment and potentially hazardous chemicals, they can risk transmitting these contaminants when they return into the uncontrolled work spaces. This can lead to disastrous results beyond the cleanroom facility, including the spread of harmful illnesses and bacterial infection. Not only will this harm employees or visitors (especially those who are immunocompromised), it will severely impact the quality of the product research or experiments being undertaken. Many studies have found that the largest source of contamination (80%) is made up from employees in the workplace. There are several ways to minimise these dangers such as using Personal Protective Equipment (PPE). While this is very important, it is just as crucial to ensure that employees understand how to follow strict protocols for wearing their sterile garments before entering the cleanroom. So what is a Sterile Gown? Sterile gowns are specially designed apparel which undergo a sterilisation process using gamma-radiation. They are examples of PPE equipment used to prevent the spread of infections, especially in health care settings where staff frequently come into contact with dangerous liquids, vapours or solid substances. Sterile Gowning Supplies Sterile gowning for cleanrooms can be purchased for one time use or reusable. If the garments are reusable, then the washing process must be validated. The following supplies must be sterile and non-shedding: Sterile Gloves. Head Covers & Protective Goggles – Head and beard coverings are necessary for complete contamination control (reduces shedding of skin flakes and hair particles). Shoe Covers & Overshoes – To prevent dirty footwear from contaminating the cleanroom and protect workers from potential chemical spillage or equipment hazards, it is necessary to wear both shoe covers and overshoes Hoods & Coveralls. Do’s & Don’ts Before Entering the Cleanroom The cleanroom gowning process should begin from when the operator leaves the house and arrives at the workplace. Here is a checklist of the essential hygiene rules: No perfume, cologne and after shave. No cosmetics (skincare and makeup). No jewellery and accessories (e.g. watches). No open-toed shoes.

According to a recent Factor survey, the global Clean Room Technology is poised to capture significant market opportunities from a variety of end-use industries. This research on the global Clean Room Technology market provides a comprehensive analysis of the different main factors influencing the market's overall performance, both positively and negatively. This study contains detailed statistics on the consumption and demand ratios of various products/services in relation to the Clean Room Technology's growth dynamics. Aside from that, the study provides accurate sales and volume statistics for all major geographic regions over the forecast period. The global Clean Room Technology report is a useful tool that offers dependable data on different facets of the industry, such as threats and opportunities. In addition, readers of this study will receive an in-depth overview of various patterns in the global Clean Room Technology, as well as technical and product innovations. The report segments the market based on various key aspects such as product form, end-use/application, and area to include an in-depth analysis of the Clean Room Technology. The study on the global Clean Room Technology includes a variety of forecasts and estimates based on primary and secondary analysis conducted by Market Reports analysts. The researchers used a variety of business intelligence methods to present reliable data on a variety of topics, including estimates and information on key facets of the global Clean Room Technology. Analysts took into account all improvements in the Clean Room Technology as a result of the COVID-19 pandemic while writing this paper. New regulations are currently being formulated by regulatory bodies from different continents, including developed and emerging countries. These rules would aid countries in dealing with the continuing macrocosmic distress caused by the COVID-19 epidemic in all of those areas. As a result, the research outlined in this report will serve as a valuable source of information on a variety of important topics, such as changing government policy as a result of COVID-19 disturbances. The information included in the global Clean Room Technology report is beneficial to key stakeholders such as market players, investors, and policymakers. This information would aid them in determining their next strategies for dealing with the effects of the recent COVID-19 pandemic while still allowing them to maintain a strong position in the Clean Room Technology business. As a result, the study is beneficial to both potential entrants and existing businesses seeking to become influential in the post-COVID period. Top Key Players Included In Clean Room Technology Market Are : Milacron (United States), Angstrom Technology (United States), Fabtech Technologies (India), Ferry Group (Hungary), Weiss Technik (Germany), Taikisha Ltd. (Japan), Integrated Cleanroom Technologies Private Limited (United States), GMP Technical Solutions Private ...

Cleanroom Technology Conference is back again for its annual two-day live event taking place in Birmingham, UK. Kicking off on 25th May 2022, the event aims to bring clarity and guidance on the latest standards, innovations and technologies in the cleanroom and controlled environment industries. The event is aimed at those working in the cleanroom and contamination control industries, providing the perfect platform to enhance your expertise, catch up with clients and industry peers, and adopt a broader and global perspective of real-time practices. Cutting-edge content for professionals The multi-sector, international event will cover a wide breadth of the industry through case studies and lectures from a panel of international experts, shedding light on industry developments from the past year, as well as future projections for all sectors. Topics covered include regulations and standards, microbiology, containment, operation and validation, consumables, clothing & PPE, GMP audits, sterilisation, cleanroom design and safe utility usage.
Categories
New Products
Cleanroom Wipers For Screen Cleaning Fiber Lint Free Cleanroom Wipes Dust Free Read More
Polyester Spunlace Double Knit Nonwoven Cleanroom Wipes Read More
Polyester Nylon Knitted Microfibre / Microdenier Wiper Read More
Direct Manufacturer Factory Selling Nonwoven Wiper Rolls Industrial Wiper Rolls Read More
Copyright © 2024 Nanan Jiedao Electronic Material Co.,Ltd.. All Rights Reserved. Powered by

IPv6 network supported